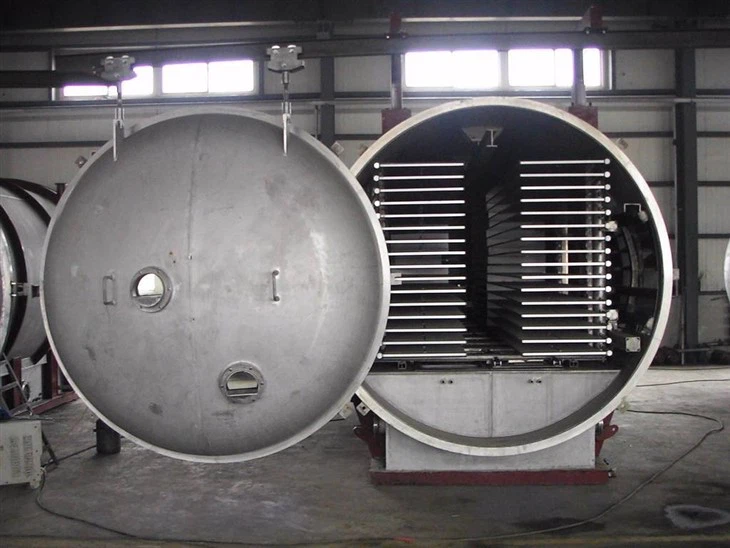
Large Freeze Dryer Machine
(a)10 series
Lab Scales Desktop(Freeze-dried material 1.5-2KG)
(b)12 series
Lab Scales Vertical(Freeze-dried material 2KG)
(c)18 series
Scientific Research Scales(Freeze-dried material 3KG)
2.Pilot Freeze Dryer:
0.2m²/0.3m²/0.5m²/1m²/2m²/---Pilot Scales(Freeze-dried material 3KG-20KG)
3.Industrial Freeze Dryer:
5㎡/10㎡/20㎡/30㎡/50㎡/100㎡/200㎡/300㎡(Freeze-dried Weight 5T~60T)
4.Customization: set up the specifications you need
(a)Freeze-dried Area
(b)Freeze-dried Weight
(c)Freeze-dried Material
(d)Interlayer Quantity/Size
(e)Cold Trap Temperature
Description
Technical Parameters
Large Freeze Dryer Machine,commonly known as lyophilizers or freeze dryers,are advanced pieces of equipment that utilize the principles of vacuum and cryogenic drying to effectively dehydrate materials while maintaining their structural integrity and biological activity.Originating from the vacuum freeze-drying technology of the 1920s,these machines have evolved significantly and are now widely used across various industries,including pharmaceuticals,biotechnology,food processing,and chemical engineering.Large freeze-dryer plays an irreplaceable role in modern industrial production with its unique working principle, wide application fields and significant advantages and characteristics.
In the pharmaceutical industry,industrial freeze dryers are used to dry sensitive drugs,vaccines,and biopharmaceuticals.In the food industry,they are used to preserve fruits,vegetables,and meats while maintaining their nutritional value and flavor.In biotechnology,they are essential for preserving cell cultures,enzymes,and other biological samples.In the chemical industry,they are used to dry powders,granules,and other solid materials.
(View other types: Laboratory ;Pilot;Industrial)
We provide Large Freeze Dryer Machine, please refer to the following website for detailed specifications and product information.
Product: https://www.achievechem.com/freeze-dryer/industrial-freeze-dryer.html
Specifications Chart

Application in Pharmaceutical Field
|
|
|
Biological agents are particularly sensitive to temperature changes and require specific storage conditions to maintain their biological activity over the long term.Dehydration of reagents reduces temperature sensitivity,driving the continued development of freeze-dried bead technology in the fields of diagnostic reagents and pharmaceuticals.
Recently, Dr.Mattia Cassanelli,technical business manager of the British Biopharma Group,introduced the application of LyoBead technology in the diagnostic and pharmaceutical fields in a webinar and introduced the application of lyophilized beads in different fields.
What are LyoBeads
LyoBeads are customized freeze-dried spheres.Each unit of feeze-dried beads contains a specific content of materials.
The most common in the pharmaceutical and diagnostic industries are PCR master mix lyophilized beads,which can be stored at room temperature for long periods of time.It is also used in microfluidics,drug delivery and feeze-drying of bacteria to meet the growing market demand.
Why use LyoBeads
1) The main benefit of feeze-dried beads is ease of use.Develop a formulation that can be used in multiple applications at different temperatures or in different containers,reducing R&D investment.
2) Freeze-dried beads are stable in nature,can be produced in high quantities on a feeze-drying machine,and can be stored in bulk before packaging.
3) After feeze-drying,the surface area of spherical feeze-dried beads is maximized,and the rehydration time can also be shortened.
Application of LyoBeads in R&D
When screening formulations for freeze-dried beads,there are three main stages of testing: compatibility,primary screening,and secondary screening.
As part of the compatibility step,it is recommended that up to 30 excipients be selected based on experience and initial thermal analysis results.These excipients are then tested to check their compatibility in the liquid state to ensure that interference does not occur.
From these initial tests,a number of promising candidate liquid formulations were selected for lyophilization.All candidate formulations were processed using the same Large freeze dryer machine.Samples were compared under the same process conditions.Characterization of optimal dry products.
In some cases,statistical analysis methods(Design of Experiments DoE)can expand the formulation candidate list to identify the best formulation based on specific parameters,such as glass transition temperature(Tg')of the active pharmaceutical ingredient(API),lyophilized beads Mechanical properties and product activity after lyophilization.Comply with regulations to define the design space and determine the optimal lyophilization conditions for each batch.
Once the LyoBead formula is developed,production can be scaled up.
Case Study
► Diagnostic reagents-PCR technology
This study aimed to extend the shelf life of a developed liquid formulation of the COVID-19 detection PCR test.Reagents need to be stored in high-throughput(96-well format)at room temperature without compromising reaction quality.Additionally,consideration needs to be given to increasing production within a short period of time.
Thermal analysis of liquid formulations using feeze-drying microscopy gradually increases the temperature to visually determine the collapse point.Further analysis using Lyotherm(BioPharma Group),combined with electrical impedance and differential thermal analysis(DTA),determined the glass transition temperature of the formulation.
Based on the above thermal analysis results,enzymes,buffers,primers,probes,etc.were combined to formulate a feeze-dried bead solution formula,and the ingredients of the formula were determined through compatibility,primary screening,and re-screening.
The morphology and fineness of the beads were visually assessed,followed by further characterization of the thermal properties of the product by modified differential scanning calorimetry(DSC).
In order to understand how the product behaves during storage,hygroscopicity and mechanical stability need to be evaluated.
Dynamic Moisture Sorption(DVS)measures how well a sample absorbs moisture at a given temperature and will indicate how long the product can be stored under characteristic conditions.
Lyophilized products are subject to mechanical stress during storage and transportation.Applying controlled pressure to the lyophilized cake using MicroPress(Biopharma Group)allows measurement of the percentage of strain that the lyophilized beads can withstand without being destroyed.Visualize this with breakpoints on the curve that indicate the product is fragile.
The most robust product is then evaluated for rehydration.Freeze-dried products can be rehydrated faster and easier because the feeze-drying process leaves tiny pores.The size,distribution,and connectivity of these pores can be examined by electron microscopy.
In conclusion,based on the above studies,several candidates for COVID19 PCR detection were tested and compared.After identifying the lead product candidates and optimizing cycling conditions,several batches were validated,confirming the lyophilized bead product for commercial use.
► Biological Products - Bacterial Vaccines
In this study,the goal was to develop a suitable formulation of an oral vaccine containing inactivated Gram-negative bacteria that is stable over the long term and allows rapid reconstitution.
Research into different formats and methods showed that lyophilized beads were ideal.The research also supports technology transfer and new production lines.
Research shows that rapid freezing of freeze-dried beads does not destroy bacteria and is the preferred method for making freeze-dried beads.After the first screening cycle,compatibility testing and post-processing analysis identified suitable lyoprotectants.
BioPharma assists in transferring the technology to the customer's site and helps create efficient operational processes on site.
► Drugs & Oral tablets
Oral tablets require grinding and compression before being placed in packaging.In this study,the objective was to trial the use of lyophilized beads for the preparation of pharmaceutical products for oral administration.The design of lyophilized beads does not require grinding and tableting,which is beneficial both technically and commercially.
Analyzing and optimizing lyophilized beads requires DOE methods to optimize parameters.This method uses several of the previously described techniques to analyze API activity,dissolution rate,thermal stability in the frozen state,and mechanical properties of dried spheres.
By calculating the residual moisture content and modulating differential scanning calorimetry(MDSC)analysis,the most suitable time for sample discharging from the freeze dryer was determined.
These studies ensured the development of efficient production of lyophilized beads,which absorb less water and are faster and more economical to produce than ground and tabletted products.
Influence of freeze-drying technology on drug prices
Production cost increase
Equipment investment
Freeze-drying technology requires specialized Large freeze dryer machine,which is often expensive and requires regular maintenance and updating.Therefore,drugs produced by feeze-drying technology will have a higher cost in terms of equipment investment.
Energy consumption
The freeze-drying process requires a large amount of energy,including electricity and refrigerants.These energy costs are also reflected in the final price of the drug.
Process complexity
Freeze-drying technology is relatively complex and requires precise control of parameters such as temperature,pressure and humidity.This increases the technical difficulty and labor cost in the production process.
Product quality and stability improvement
Maintenance of Active ingredients
Freeze-drying is an ideal way to maximize the retention of active ingredients in pharmaceuticals,especially for those that are heat sensitive.
Extended shelf life
Pharmaceutical products produced through feeze-drying technology have a longer shelf life,which reduces the loss of pharmaceutical products during storage and transportation,which may reduce the overall cost(although the initial investment is higher).
Improved bioavailability
Freeze-dried drugs generally have better solubility and bioavailability,which helps improve drug efficacy and patient compliance.
Market demand and price strategy

Market demand
With the increasing demand for high quality and high stability drugs,drugs produced by lyophilization technology may be favored by the market.This could lead to drug prices rising to reflect their higher quality and value.
Pricing strategy
Pharmaceutical companies consider a variety of factors when setting prices,including production costs,market demand,and competition.Therefore,even if the adoption of feeze-drying technology increases production costs,pharmaceutical companies may adjust their pricing strategies to remain competitive.

Policy and regulatory impact
Policy support:
Some countries or regions may introduce policies to support pharmaceutical enterprises that adopt advanced production technologies(such as freeze-drying technology)to encourage technological innovation and industrial upgrading.These policies may include tax incentives,financial subsidies,etc..
Regulatory requirements:
Pharmaceutical production needs to meet strict regulatory requirements,including GMP(Good Manufacturing Practice).Pharmaceutical products manufactured using lyophilization technology need to meet higher quality standards,which can increase production costs and regulatory costs.
Conclusion
In the pharmaceutical and diagnostic fields, large freeze dryer machine beads are a simple solution for creating stable products that can be stored for long periods of time.Once produced,the beads can be placed into any container to accommodate different product variations and can be stored in bulk before packaging.However,there are different ways to create these beads,which need to be optimized through compatibility testing,primary screening,and secondary screening.
LyoBeads can be used in many different areas,several of which are described in the case studies above.One of the most important applications of these beads is in PCR,where stable lyophilized products stored at room temperature can accelerate and increase the throughput of PCR screening assays.
Analytical drying
● Analyze the purpose of drying
After the sublimation drying stage, there may still be some residual bound water or adsorbed water in the chemical raw materials, which exists in the raw materials in the form of chemical combination or physical combination. The purpose of analytical drying is to remove these residual moisture, so that the raw material to achieve the final drying effect.
● Analyze the principle of drying
The principle of analytical drying is to increase the activity of water inside the raw material by further increasing the temperature, so that it can be more easily evaporated from the raw material. Under vacuum conditions, these water molecules are quickly removed, thus achieving the purpose of drying.
Analyze the drying process
Heating up
After sublimation drying, gradually increase the temperature in the drying room, so that the residual water inside the raw material begins to evaporate.
Maintaining vacuum
The vacuum in the drying chamber needs to be maintained throughout the analytical drying process to ensure that water molecules can be smoothly evaporated from the raw material and pumped away.
Monitoring and adjustment
Real-time monitoring of the temperature, vacuum degree and other parameters in the drying room through the control system, and adjustment according to the actual situation to ensure the stability and efficiency of the analytical drying process.
Factors affecting the analytical drying effect
Temperature
Temperature is one of the key factors affecting the analytical drying effect. Too high a temperature may lead to the destruction of the heat-sensitive components in the raw material, while too low a temperature will prolong the drying time.
Vacuum degree
The higher the vacuum degree, the easier it is for water molecules to evaporate from the raw material and be pumped away. Therefore, maintaining a high vacuum degree is an important means to improve the analytical drying efficiency.
Raw material properties
Raw materials with different properties have different adaptability to dry conditions. For example, some raw materials may have higher hygroscopicity and require longer analytical drying times to remove residual moisture.
Precautions

Avoid overheating
During the analytical drying process, it is necessary to avoid excessive temperature resulting in the destruction of heat-sensitive components in the raw material. Therefore, it is necessary to choose the appropriate temperature range according to the nature and requirements of the raw material.

Maintaining vacuum
The vacuum in the drying chamber needs to be maintained throughout the analytical drying process to ensure that water molecules can be smoothly evaporated from the raw material and pumped away. This requires the vacuum system to have efficient and stable working performance.

Real-time monitoring
Real-time monitoring of the temperature, vacuum degree and other parameters in the drying room through the control system, and adjustment according to the actual situation to ensure the stability and efficiency of the analytical drying process.
Preservation method suggestion
Storage container
Choose a container with good sealing: The freeze-dried material should be stored in a closed container to prevent air, moisture, dust and other pollution and corrosion of the material. It is recommended to use a plastic bag, glass bottle or aluminum foil bag with good sealing performance for storage.
Container cleaning: The storage container should be cleaned before use, and ensure that the inside is dry and free of moisture.
Preserve the environment
Cryogenic storage
The freeze-dried material should be stored at a low temperature, and it is generally recommended to store it in the refrigerator or freezer. If it needs to be stored for a long time, it is recommended to store the material in a freezer below minus 20 ° C.
Storage away from light
Light may adversely affect the material, so it is recommended to store the material in a dark place, such as a drawer or an airtight cabinet.
Dry environment
Materials should be placed in a dry environment, avoid contact with water or wet ground, to prevent moisture resulting in mildew and deterioration.
Storage conditions
Avoid high temperatures
High temperatures may cause material quality to decline, so avoid direct sunlight and high temperatures.
Avoid extrusion
Materials should be placed on a flat ground to avoid stacking or being squeezed to prevent damage or quality degradation.
Note the expiration date
Pay attention to the expiration date when storing materials. Materials beyond the expiration date may deteriorate or fail, so it is not recommended to use.
Can freeze-dried materials be used directly?
The freeze-dried material can be used directly in most cases, but the specific situation needs to be determined according to the nature and use of the material and the quality after drying.
Freeze-drying is a drying method that removes water from materials through the principle of sublimation, and its main advantage is that it can maintain the original characteristics of the material, such as activity and structure. In the freeze-drying process, the material is first frozen into a solid state and then heated under vacuum conditions to sublimate the ice crystals directly into water vapor, thereby removing the water. This method avoids deformation, deterioration and other problems caused by temperature changes in the drying process of materials, so it is widely used in pharmaceutical, food, biological materials and other fields.
For most materials, freeze-dried can be used directly. For example, in the pharmaceutical industry, freeze-dried pharmaceutical products can often be used directly for clinical use or further processing. In the food industry, freeze-dried food can maintain its original taste and nutritional value, and is easy to store and transport. In the field of biomaterials, freeze-dried materials can maintain their biological activity and structural stability for cell culture, tissue engineering and other applications.
However, in some special cases, the freeze-dried material may require additional processing before it can be used. For example, if the material has agglomeration, caking and other problems during the freeze-drying process, it may need to be dispersed by grinding, screening and other methods. If the material is sensitive to humidity, it may need to be sealed and packaged immediately after drying to prevent moisture absorption. In addition, for some specific uses of materials, such as certain biological products or chemical reagents, may need to undergo strict quality testing before use.
Hot Tags: large freeze dryer machine, China large freeze dryer machine manufacturers, suppliers, factory, Double Jacketed Glass Reactor, Rotating Evaporator, 5l Rotovap, PPL Lined Hydrothermal Autoclave, Rotovap Machine, Complete Short Path Distillation Kit
Previous
Deep Freeze DryerSend Inquiry